PET business
NARD Institute's PET business.
NARD Institute,Ltd. was established in 1972 by creating a new business model for contract research. We began the synthesis of specially ordered PET reagents in 1995, and have accumulated the experiences of many synthetic precursors and referential standard compounds.
Furthermore, in 2006, we entered into a business arrangement with PharmaSynth AS Inc. (Estonia). Since then, we have been distributing PET reagents produced by both the companies in the global market. Both the companies have been currently distributing PET/SPECT precursors as well as referential standard compounds for general use.
* PharmaSynth AS products are exclusively sold in Japan by NARD Institute,Ltd.
Sales of PET reagents
We have a wide range of products in our inventory. The minimum amount for ordering is 10 mg. In case of majority of our products, purity is higher than 98% (or 95%). We can accommodate rapid delivery upon request, and products will be provided with a certification of analysis (HPLC charts). Please feel free to contact us.
* Our products are precursors and referential standard compounds and are not radio-labeling compounds.

Chemical List (precursors & referential standard compounds)
PET chemicals
- Acetylcholine :
- NP034-0 : (+)3-MPB
NP034-1 : (+)3-PB
NP035-0 : (-)3-MPB
NP035-1 : (-)3-PB
NP059-0 : (+)3-EPB
NP060-0 : (+)3-PPB
NP042-0 : Donepezil
NP042-1 : Desmethyl-donepezil
NP072-0 : MP4A
NP072-1 : P4A
NP073-0 : MP4P
NP073-1 : P4P
PP089-0 : Nicotine
PP089-1 : Nornicotine
- Mitochondrial Complex-I (MC-I) :
- NP087-0 : BCPP-EM
NP087-1 : Desmethyl-BCPP-EM
NP088-0 : BCPP-EF
NP088-1 : Tosyl-BCPP-EF
- Adenosine A2A :
- PP027-0 : TMSX
PP027-1 : Desmethyl-TMSX
PP090-0 : SCH442416
PP090-1 : Desmethyl-SCH442416
- Translocator protein (TSPO) :
- PP010-0 : (R) –PK11195
PP010-1 : (R)-N-Desmethyl PK11195
PP091-1 : (S)-N-Desmethyl PK11195
PP092-0 : ER176
PP092-1 : Desmethyl-ER176
PP011-0 : Flumazenil
PP011-1 : Desmethyl-flumazenil
PP011-2 : Nitro-flumazenil
PP046-0 : PBR 28
PP046-1 : O-Desmethyl-PBR 28
NP065-0 : DAA1106
NP065-1 : Desmethyl-DAA1106
NP066-0 : FEDAA1106
NP067-0 : AC-5216
NP067-1 : Desmethyl-AC-5216
NP068-0 : FEAC
NP069-0 : DAC
NP069-1 : Desmethyl-DAC
NP070-0 : FEDAC
PP079-0 : RO-15 4513
PP079-1 : RO-44 3902
PP080-0 : DPA-713
PP080-1 : Desmethyl-DPA-713
PP082-0 : DPA-714
PP082-1 : Tosylate-DPA-714
- Dopamine :
- PP003-0 : 6-Fluoro-L-DOPA
NP003-1 : Bpin-L-DOPA
PP003-2 : 4-O-Pivaloyl-L-DOPA
PP003-3 : Sn-L-DOPA
PP004-0 : (R)-(+)-SCH-23390
PP004-1 : SCH-24518
PP093-1 : (S)-SCH-24518
PP005-0 : Raclopride
PP005-1 : Desmethyl-raclopride
PP006-0 : FLB 457
PP006-1 : FLB 604
PP007-0 : NNC 112
PP007-1 : Desmethyl-NNC 112
PP008-0 : Fallypride
PP008-1 : Tosyl-fallypride
PP019-0 : β-CFT
PP019-1 : nor-β-CFT
PP019-2 : β-CFT-acid
PP020-0 : β-CIT
PP020-1 : nor-β-CIT
PP022-0 : PE2I
PP022-1 : Desmethyl-PE2I
PP045-0 : Fluoroethyl-PE2I
PP045-1 : Tosylethyl-PE2I
PP094-0 : p-Me-β-CPT
PP094-1 : p-Me-β-CPT-acid
PP094-2 : p-Me-nor-β-CPT
PP095-0 : MCL-524
PP095-1 : MCL-556
PP096-0 : N-Methylspiperone
PP096-1 : Spiperone
- Histamine :
- PP033-0 : Doxepin
PP033-1 : Desmethyl-Doxepin
- Amyloid :
- NP039-0 : BF-227
NP039-1 : THK-001
PP043-0 : 6-MeO-BTA-0
PP043-1 : 6-MOMO-BTA-0
PP044-0 : 6-OH-BTA-1
PP044-1 : 6-OH-BTA-0
NP115-0 : ABC115
NP115-1 : Tosyl-ABC115
- Tau :
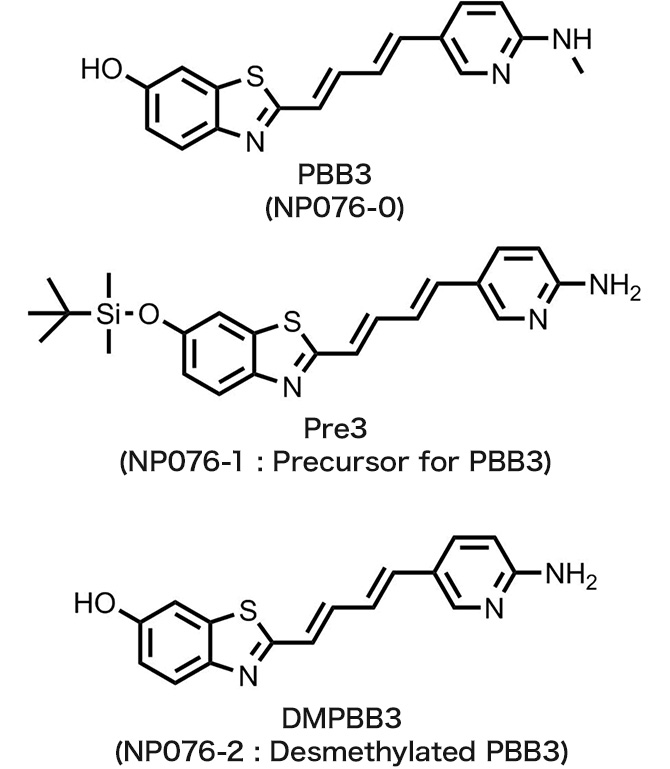
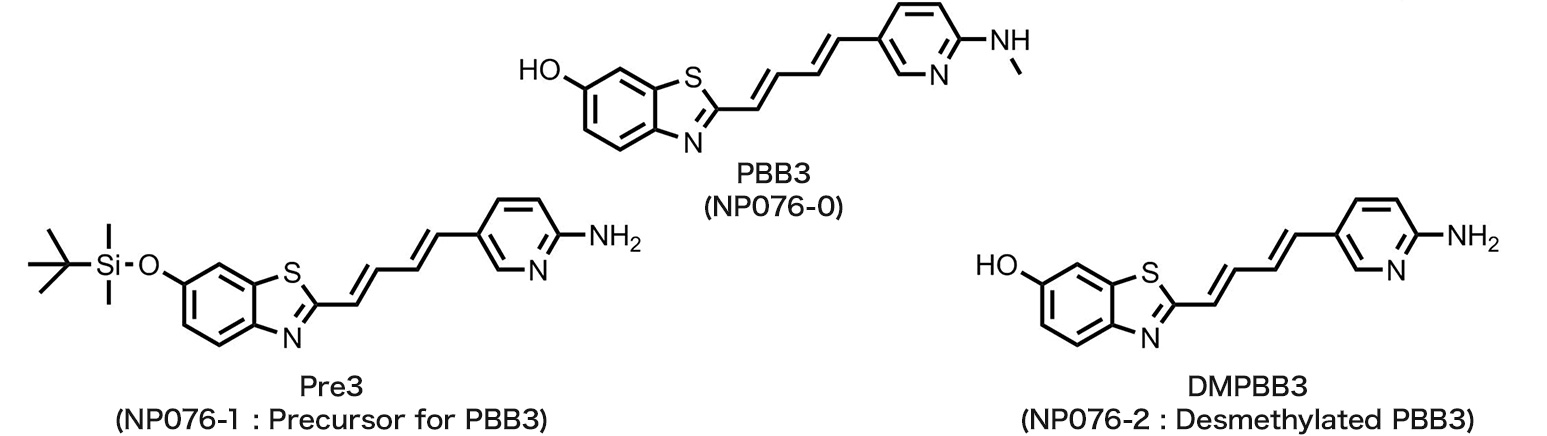
- NP076-0 : PBB3
NP076-1 : Pre3
NP076-2 : DMPBB3
- Androgen receptor :
- PP097-0 : 16β-FDHT
PP097-1 : 16α-TfO-EDA
PP098-0 : 16α-FDHT
- Aromatase :
- PP048-0 : Vorozole
PP048-1 : Desmethyl-Vorozole
- Amino Acid :
- PP016-1 : L-Homocysteine thiolactone HCl
PP058-0 : D-Methionine
PP058-1 : D-Homocysteine thiolactone HCl
PP018-0 : L-2-Fluoro-tyrosine
PP018-1 : 2-SnT
NP050-0 : FAMT
NP050-1 : AMT
NP053-0 : FMT
NP053-1 : 2-SnMT
NP075-0 : L-FBPA
NP061-1 : L-BPA
PP099-0 : FSPG
PP099-1 : Nitrotosyl-SPG
- Estrogen :
- NP071-1 : MMSE
- Choline :
- PP101-0 : Fluorocholine bromide
PP101-1 : Dimethylaminoethanol
PP102-0 : Fluoroethylcholine chloride
- Fatty acid :
- NP100-0 : FTAP1
NP100-1 : Tosyl-FTAP1
PP103-0 : FPIA
PP103-1 : TsO-PIAMe
- Hypoxia :
- NP036-0 : FMISO
NP036-1 : NITTP
NP051-0 : FAZA
NP051-1 : Tosyl-FAZA
NP052-0 : CuATSM
NP052-1 : ATSM
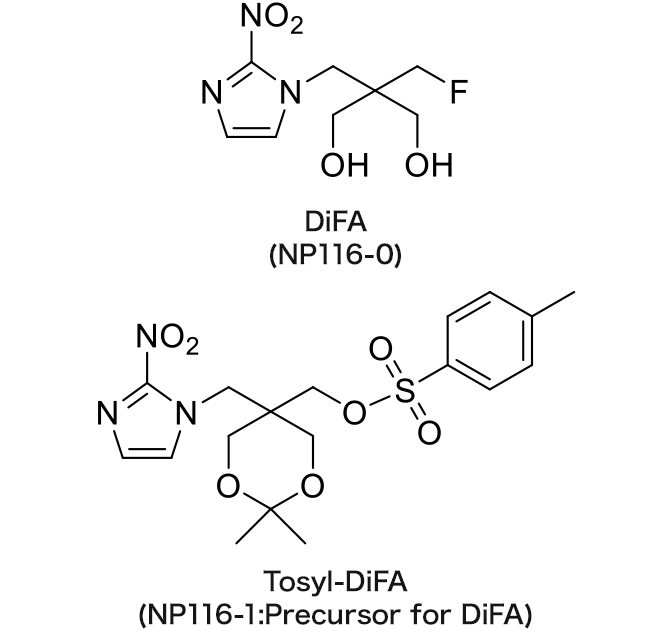
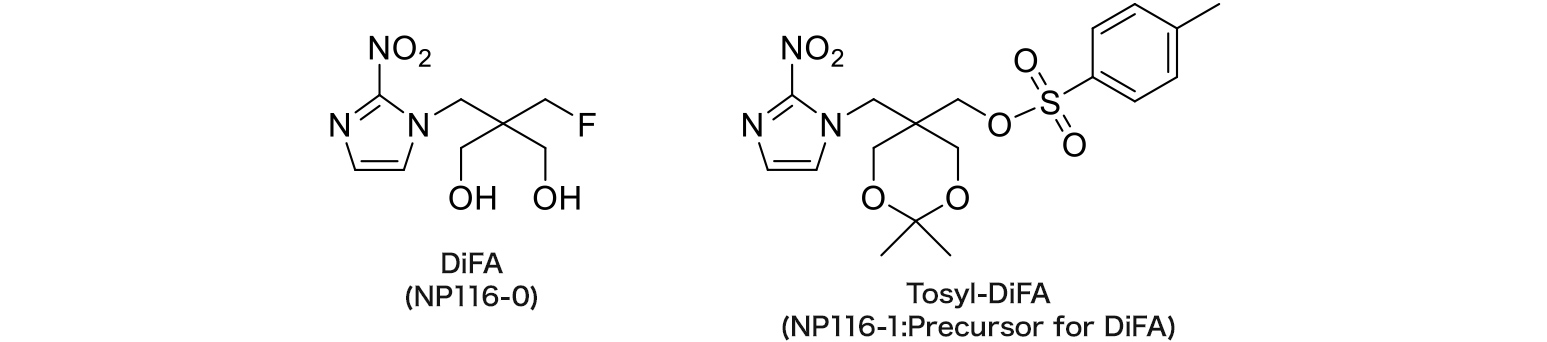
NP116-0 : DiFA
NP116-1 : Tosyl-DiFA
- Norepinephrine :
- PP031-0 : MeNER
PP032-0 : FD2-MeNER
PP032-1 : NER
- µ-Opioid :
- PP017-1 : Desmethyl carfentanil
- DNA synthesis :
- NP002-0 : FLT
NP002-1 : Anhydrothymidine
NP002-2 : Boc-thymidine
PP002-3 : DB-thymidine
NP049-0 : 4DST
NP049-1 : Sn-4DST
- Glucose Metabolism :
- NP105-0 : L-FDG
NP105-1 : L-mannose triflate
- Metabotropic glutamate receptor 5 (mGluR5) :
- PP106-0 : FPEB
PP106-1 : Nitro-PEB
- Adreno-cortical tissue :
- PP081-0 : Fluoroetomidate
PP081-1 : p-Cl-Tosyletomidate
PP106-0 : Etomidate
PP106-1 : Desethyletomidate
- Serotonin (5HT) :
- PP012-0 : Altanserin
PP012-1 : Nitro-Altanserin
PP013-0 : Setoperone
PP013-1 : Nitro-Setoperone
PP014-0 : MDL100907
PP014-1 : Desmethyl-MDL100907
PP015-0 : WAY100635
PP015-1 : WAY100634
PP025-0 : DASB
PP025-1 : Desmethyl-DASB
PP026-0 : MADAM
PP026-1 : N-Desmethyl-MADAM
NP037-0 : (+)-McN5652
NP037-1 : pre-(+)-McN5652
NP038-0 : (-)-McN5652
NP038-1 : pre-(-)-McN5652
PP078-0 : AZ10419369
PP078-1 : N-Desmethyl-AZ10419369
PP083-1 : N-Boc-Desmethyl-CIMBI-36
- Vesicular monoamine transpoter2 :
- PP040-0 : FE-(+)-DTBZ
PP107-0 : FCD2CD2-DTBZ
PP041-0 : (+)-DTBZ
PP041-1 : (+)-9-O-Desmethyl-DTBZ
- cannabinoid (CB-1) :
- PP047-0 : MePPEP
PP047-1 : Desmethyl-MePPEP
PP108-0 : FMePPEP
- P-glycoprotein :
- NP054-0 : (+)-Verapamil
NP054-1 : (+)-Norverapamil
NP055-0 : (-)-Verapamil
NP055-1 : (-)-Norverapamil
- Synaptic vesicle glycoprotein 2A :
- PP086-0 : UCB-J
PP086-1 : BF3-Dm-UCB-J
PP086-2 : Br-Dm-UCB-J
PP086-3 : 3-Br-UCB-J
PP086-4 : 3-Me3Sn-UCB-J
PP109-0 : SDM-8
PP109-1 : Me3Sn SDM-8
- Phosphodiesterase-4(PDE4) :
- NP056-0 : (S)-(+)-Rolipram
NP056-1 : (S)-(+)-Desmethyl-Rolipram
NP057-0 : (R)-(-)-Rolipram
NP057-1 : (R)-(-)-Desmethyl-Rolipram
- Phosphodiesterase 10A (PDE10A) :
- PP084-0 : F-MNI-659
PP084-1 : TsO-MNI-659
- NMDA :
- PP074-0 : CNS5161
PP074-1 : Desmethyl-CNS5161
- Imidazoline 2 binding site (I2BS) :
- PP085-0 : BU99008
PP085-1 : BU99007
- Neurokinin 1 receptor :
- PP110-0 : FE-SPARQ
PP111-0 : F-SPARQ
PP112-0 : GR 205171
PP110-1 : SPARQ
Ligands for SPECT imaging
- PS001-0 : Epidepride
PS001-1 : Tributyltinyl-epidepride
PS002-1 : Trimethyltin-β-CIT
PS003-1 : Tributyltin-PE2I
PS004-0 : ADAM
PS004-1 : Tributyltin-ADAM
PS005-0 : NNC 13-8241
PS005-1 : Trimethyltin-NNC 13-8241
PS005-2 : Bromo-NNC 13-8241
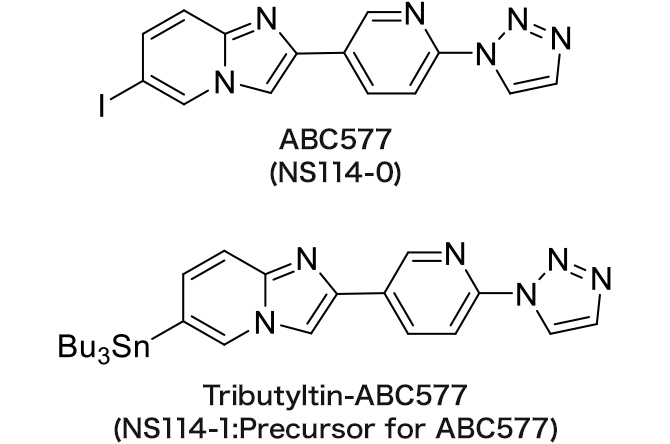
NS114-0 : ABC577
NS114-1 : Tributyltin-ABC577
Chemicals for Synthesize
- NC001-0 : Fluoromethyl tosylate
NC002-0 : Fluoroethyl tosylate
NC003-0 : LAH 0.1M-0.5mL
NC003-1 : LAH 0.1M-1mL
NC003-2 : LAH 0.25M-0.5mL
NC004-0 : 2-Bromoethyl triflate
NC005-0 : TABT
NC006-0 : HI 1mL
NC007-0 : 3-FP-6-Me-Tetrazine
NC007-1 : 3-TsOPr-6-Me-Tetrazine
NC008-1 : HMPAO
NC009-0 : NBP
Custom Synthesis
We are able to offer specially ordered compounds for PET/SPECT. For any reagent that is not listed in the catalogue, please feel free to contact us.
GMP-compliant manufacturing
We have a track record of manufacturing many PET reagents (mainly precursors) under GMP standards. Our subsidiary, NARD Chemicals Ltd., has a quality assurance system in accordance with GMP and in charge of manufacturing.
Collaborative development
By accelerating the designing of compound structures suitable for PET probes and synthesis of derivatives, we contribute to the early-stage development of PET probes.
Topics (All compounds below are available exclusively from NARD Institute, Ltd.)
DiFA
Hypoxia imaging agent
The referential standard and precursor for DiFA can be purchased under a license agreement with Nihon Medi-Physics Co., Ltd.
DiFA is a new FMISO-based derivative with stronger hydrophilicity, and has rapid clearance from urine after injection.
ABC577 (SPECT imaging)
Amyloid imaging agent
The referential standard and precursor for ABC577 can be purchased under a license agreement with Nihon Medi-Physics Co., Ltd.
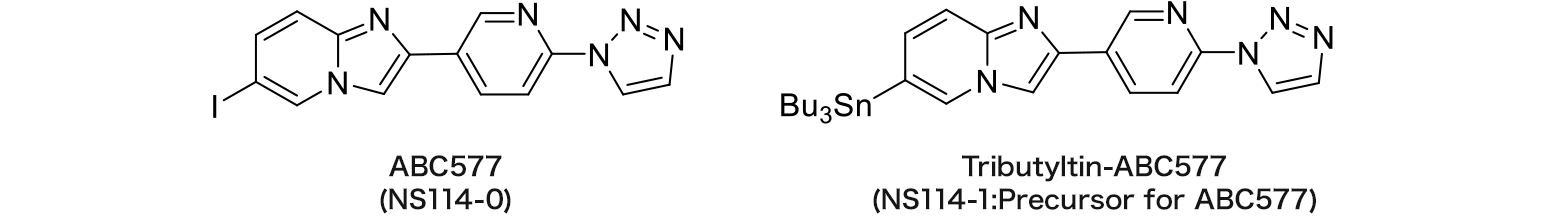
In vitro ARG of AD frontal lobe sections

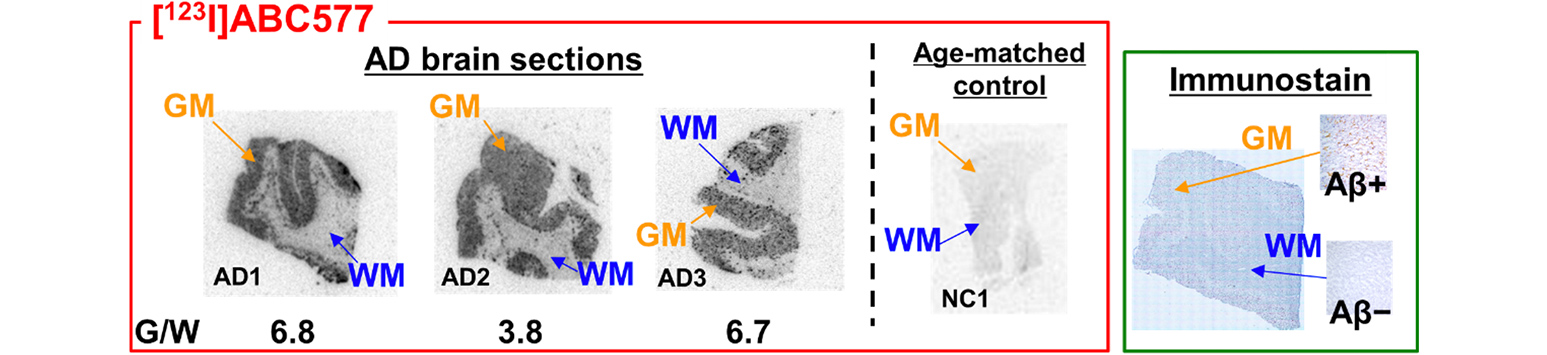
ABC577 was tested with sections from three different AD patients, AD1,2,3, and plus one age-matched control section. ABC577 clearly demonstrated specific binding to amyloid plaques, and also showed very low nonspecific binding to white matter, the gray matter to white mater ratio is from 3.8 to 6.8.
Yoshifumi Maya et al. Preclinical properties and human in vivo assessment of 123I-ABC577 as a novel SPECT agent for imaging amyloid-β. BRAIN 2016: 139; 193–203.
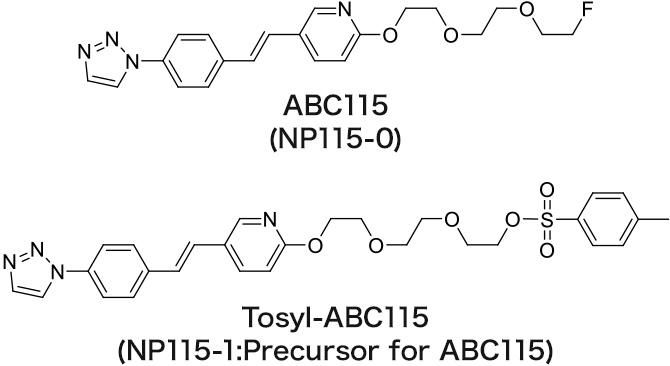
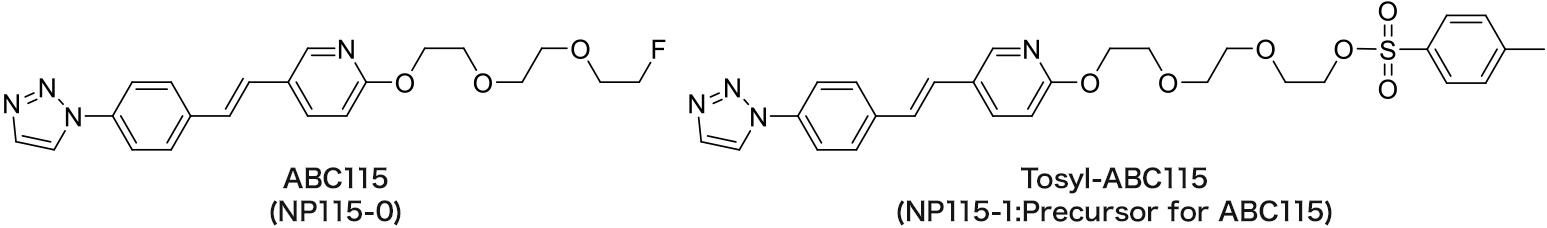
ABC115
Amyloid imaging agent
The referential standard and precursor for ABC115 can be purchased under a license agreement with Nihon Medi-Physics Co., Ltd.
The contrast ratio of gray matter to white matter of ABC115 is higher than that of AV45 and AV1, and the specific accumulation in amyloid is high.
FTAP1
Fatty acid binding protein 4 (FABP4) imaging agent
The referential standard and precursor for FTAP1 can be purchased under a license agreement with Nihon Medi-Physics Co., Ltd.
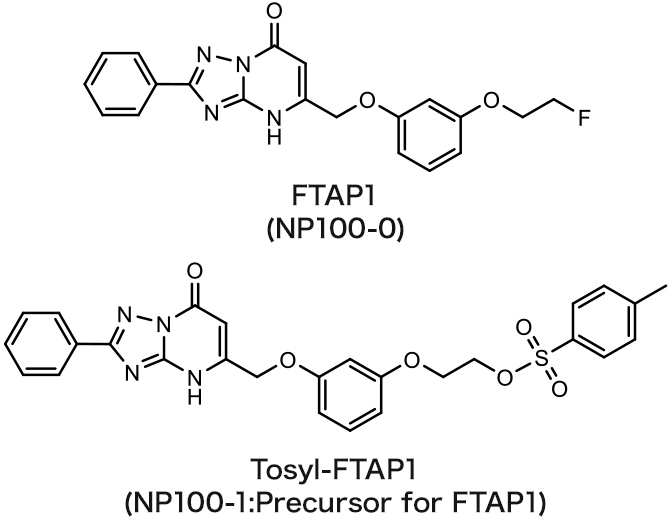
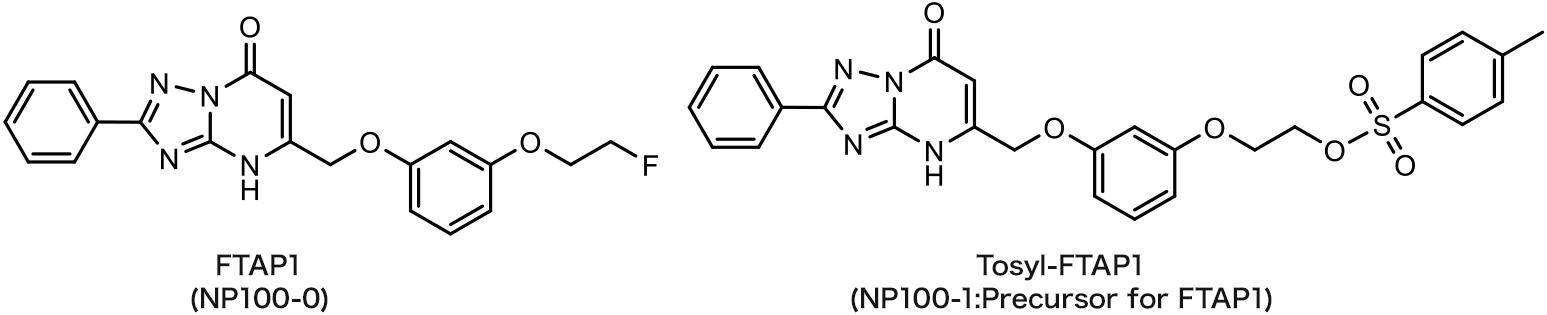
Nuclear Medicine and Biology 42 (2015) 184-191
*Watanabe et al. EJNMMI Research (2019) 9:60
BCPP-EF & BCPP-EM
MC-I (Mitochondrial complex I) imaging agent
The referential standards and precursors for BCPP-EF and BCPP-EM can be purchased under a license agreement with Hamamatsu Photonics K.K. (HPK).The BCPP-EF and BCPP-EM, which target MC-I, have been studied in various cases such as stroke, Parkinson's disease, and Alzheimer's disease that show deficiencies in MC-I activity.
* If you wish to use [18F]BCPP-EF in a clinical PET examination, we can provide its safety data in agreement with HPK. (Safety data are available for a fee.)
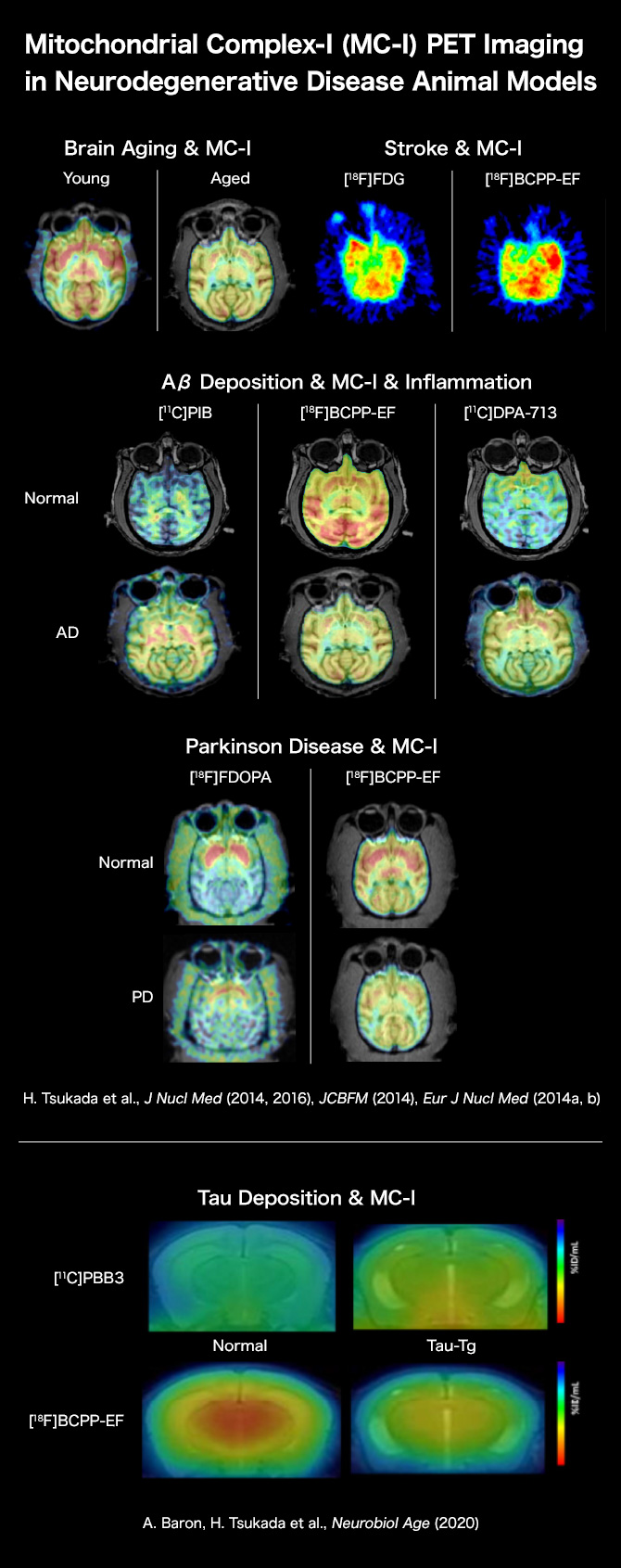
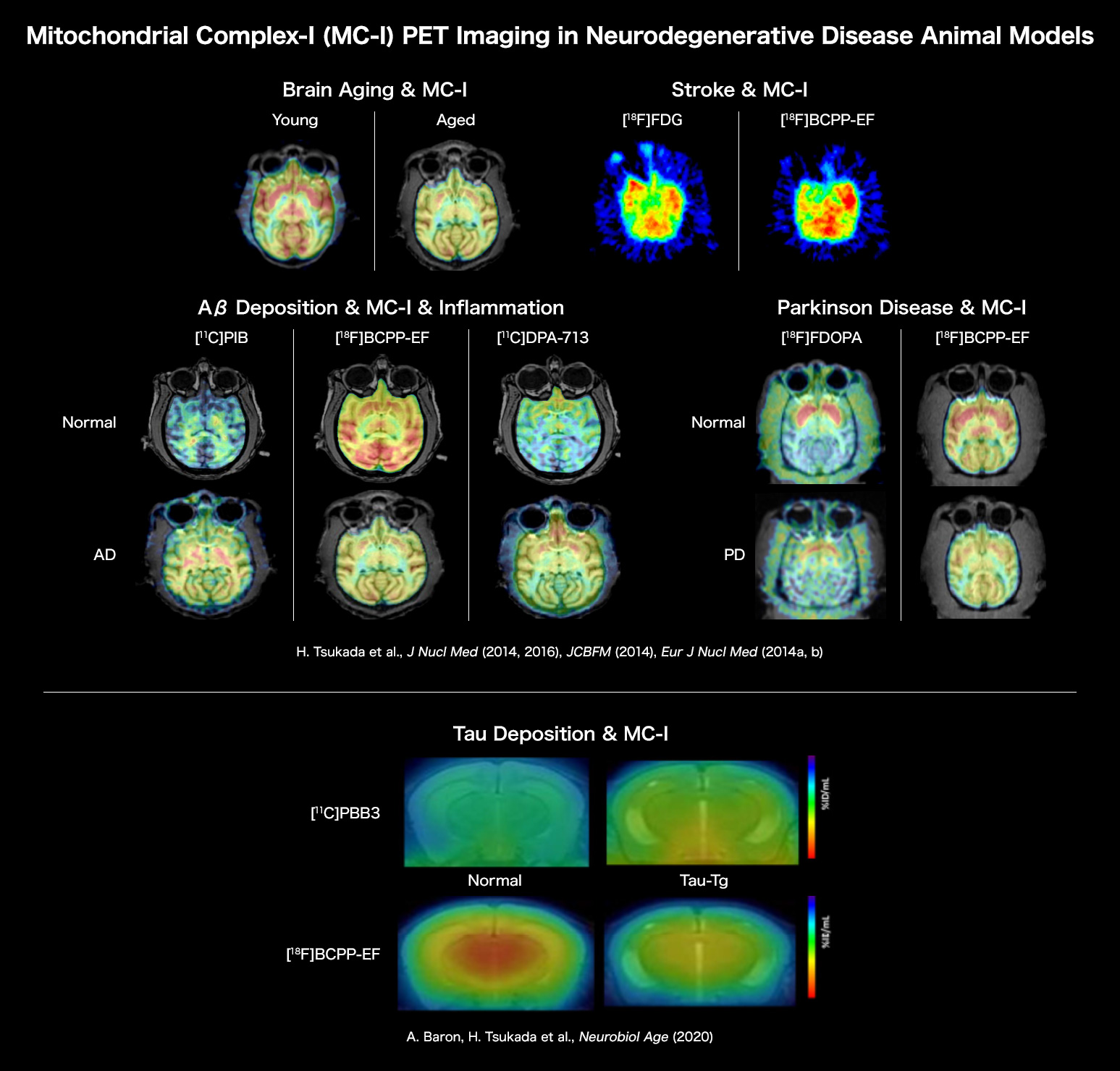
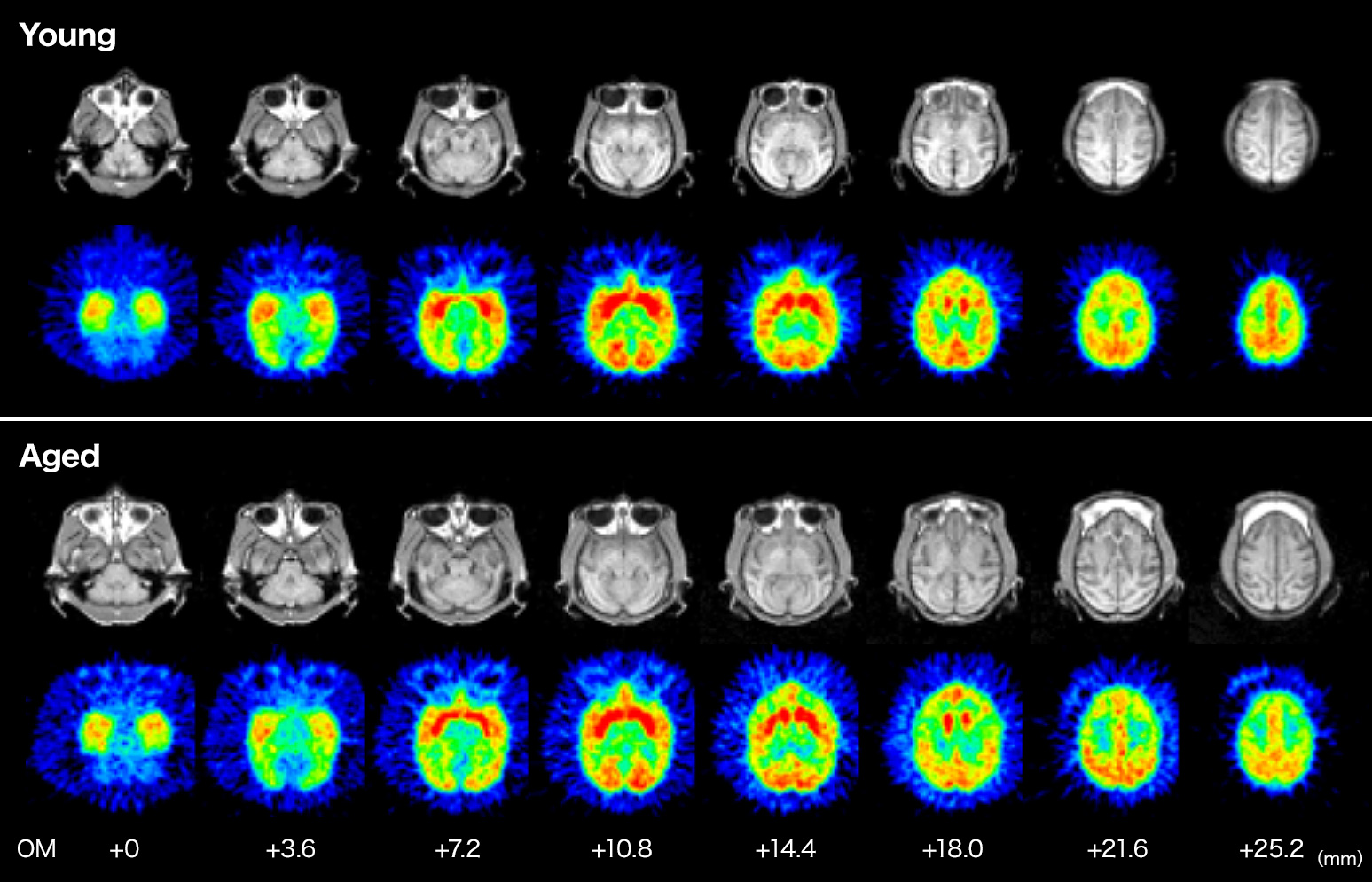
Brain Aging & MC-I
PET imaging using [18F]BCPP-EF demonstrated that mitochondrial complex-I (MC-I) activity was significantly lower in aged monkey (corresponding to ca. 80 years old in human) than young one (corresponding to ca.20 years old in human).
H. Tsukada, H. Ohba, M. Kanazawa, T. Kakiuchi, N. Harada. Evaluation of 18F-BCPP-EF for mitochondrial complex I imaging in conscious monkey brain using PET. Eur J Nucl Med Mol Imaging. 2014; 41: 755-763.
Aβ Deposition & MC-I & Inflammation
It was shown that the aging process induced amyloid-β deposition even in the brains of some part of monkeys. PET imaging using [11C]PiB for amyloid-β deposition, [18F]BCPP-EF for MC-I, and [11C]DPA-713 for neuroinflammation revealed that monkeys with higher amyloid-β deposition showed lower MC-I activity and higher neuroinflammation than those with lower amyloid-β deposition.
H. Tsukada, S. Nishiyama, H. Ohba, M. Kanazawa, T. Kakiuchi, N. Harada. Comparing amyloid-β deposition, neuroinflammation, glucose metabolism, and mitochondrial complex I activity in brain: A PET study in aged monkeys. Eur J Nucl Med Mol Imaging. 2014; 41: 2127-2136.
Parkinson Disease & MC-I
In the brains of Parkinson model monkeys created by MPTP, a dopamine-specific toxin, the impaired degrees of MC-I activity assessed using [18F]BCPP-EF were significantly correlated with those of dopamine synthesis rate measured using [18F]FDOPA.
H. Tsukada, M. Kanazawa, S. Nishiyama, N. Harada, T. Kakiuchi. PET imaging of mitochondrial complex I with 18F-BCPP-EF in brain of MPTP-treated monkey. J Nucl Med. 2016; 57: 950-953.
Stroke & MC-I
In the monkey brain after ischemic/reperfusion insult, PET imaging using [18F]BCPP-EF and [18F]FDG indicated the decreased MC-I activity and increased glucose metabolism. The later was implied that activated microglia gathering around damaged and inflammation area uptake [18F]FDG, which misleading to false-positive diagnose.
3-MPB
muscarinic receptor imaging agent
in the Monkey Brain as Measured by [11C] (+)3-MPB

PBB3
tau imaging agent
We are proud to announce that we have commercialized PBB3,* Pre3,* and DMPBB3* under an exclusive licensing agreement with APRINOIA Therapeutics Inc. Our company provides these reagents for research applications hoping to contribute to the society by supporting studies on tau diseases.
* Developed by National Institutes for Quantum Science and Technology.
[11C]PBB3 ([11C]pyridinyl-butazienyl-benzothiazole; IUPAC name, 2-((1E,3E)-4-(6-([11C]methylamino)pyridin-3-yl)buta-1,3-dienyl)benzo[d]thiazol-6-ol) is a radioactive compound applicable to positron emission tomography (PET) imaging of tau protein aggregates in living brains.1) Tau lesions, as exemplified by neurofibrillary tangles in Alzheimer’s disease (AD), are characteristic of diverse neurodegenerative dementias, and are known to tightly correlate with neuronal death in these illnesses. [11C]PBB3 reacts with tau deposits in AD with high affinity (KD = 2.5 nM) and selectivity (tau : Aβ = 120 : 1) according to an autoradiographic binding assay.1) Using an established radiolabeling protocol,2) [11C]PBB3 can be synthesized with reasonably high yield, and has been demonstrated to visualize tau pathologies in AD and non-AD dementias.1) An exploratory clinical PET study also indicated the capability of [11C]PBB3 in pursuing tau accumulation spreading in progression from prodromal to advanced stages of AD.1) Moreover, [11C]PBB3 binds to tau inclusions in transgenic mouse models of tau pathologies,1) enabling translational research and development of diagnostic and therapeutic drug candidates targeting tau lesions. To avoid conversion to its photoisomers, radiosynthesis and injection of [11C]PBB3 should be performed in a dimly lit facility.2)
References
1) Maruyama et al. Neuron 79, 1094 – 1108, 2013.
2) Hashimoto et al. J Nucl Med 55, 1532-1538, 2014.
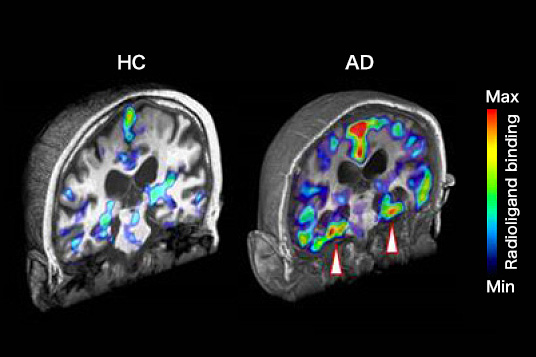
Legend for figure
Coronal PET images of brains healthy control (HC) and patient with Alzheimer's disease (AD) at 30 – 70 min after intravenous administration of [11C]PBB3. PET data are superimposed on individual 3D MRI anatomical maps. Intense radiosignals are observed in tau-rich regions including the bilateral hippocampal formations (arrowheads) of the AD brain. Images provided by National Institute of Radiological Sciences.
NARD strongly promotes research and development in PET.
Related contents
Inquiries about products and technologies
Please contact us for consultations and inquiries regarding products and technologies.