Contract synthesis and purification of highly active pharmacological substances
Along with advancements in medical sciences, the aging of society has created an unprecedented need for improving the health and quality of life.
Even though medications for lifestyle diseases are extensively available, an unfulfilled requirement is treatment for various types of cancers; such anticancer drugs are currently being actively developed. However, from the viewpoint of research and development as well as manufacturing, drugs that are potent in small doses can cause health damage. We began using a containment facility in April 2016 to ensure that such substances are handled in a safe and secure environment.
Based on the combination of our knowledge and years of organic synthesis experience within this facility, we contribute to improving the speed of drug development within this area by providing contract synthesis and purification services for highly active pharmacological substances in a safe and secure manner.




Basic policy for the containment of highly active pharmacological substances
NARD Institute Ltd. contributes to the research, development, and creation of highly potent pharmaceuticals using a newly constructed high-level containment facility as part of its contract synthesis services for chemical compounds.
In addition, we strive to minimize the occupational health and safety issues by actively promoting, establishing, and maintaining a safe and healthy work environment for our employees.
| Volume handled | Management classification of exposures | Containment management |
|---|---|---|
| - 100g | OEL 0.1 - 1µg/m3 (OEB5) | SOP/Exposure sampling |
Note: This is not a GMP-compliant facility. β-lactam antibiotics, compounds containing viable bacteria, or hormones containing steroid nucleus cannot be handled.
Facility overview
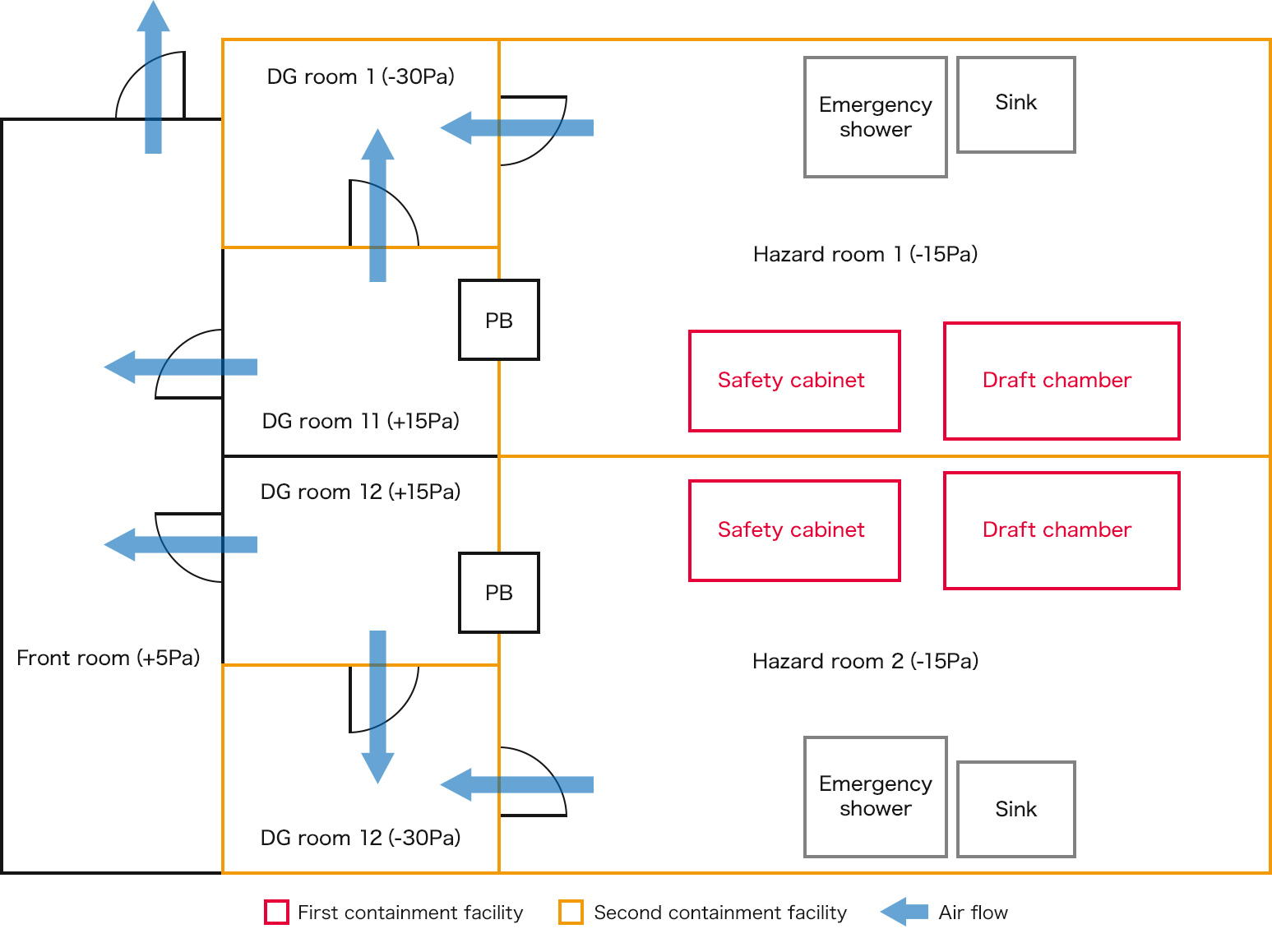

| Production room | 17m2 (2 hazard rooms) and DG room as well as pass box |
|---|---|
| Containment facility | Hazard room, safety cabinet, draft chamber |
| Static pressure control | -15Pa to -30Pa |
| Production environment, external environment | Class 100K HEPA performance, HEPA exhaust |
Related contents
Inquiries about products and technologies
Please contact us for consultations and inquiries regarding products and technologies.