Thio-tag™ Technology
Thio-tag is a mononuclear zinc complex that traps thiol compounds, which was developed by the Functional Molecular Science Laboratory, Graduate School of Biomedical and Health Sciences, Hiroshima University.
Thiol stabilization buffer

Thio-tag™ chloride protects the SH-containing compounds, such as the cysteine thiol groups, at physiological pH and room temperature under atmospheric air and id used as a selective stabilizer for analysis.
It is easily displaced under acidic conditions (pH 3) or by EDTA treatment.
| nTTB-T02 |
|---|
| 1g 12,000 JPY |
Stability of the Cys compounds
Thiol affinity chromatography

| The thiol compounds can be separated and refined at a physiological pH |
| The trapped thiol is trapped at the µmol level |
| Thiolate-trapping capacity is 10,000 times that of the carboxylate ions |
| A simple operation allows for high efficiency → operation time is less than 15 minute |
| nAG1-T01 [Thiol trapping capacity: 1µmol/mL-gel] |
|---|
| 10µL-gel x8 18,000 JPY |
| nAG2-T01 [Thiol trapping capacity: 3.5µmol/mL-gel] |
| 10µL-gel x8 18,000 JPY |
Also available in bulk
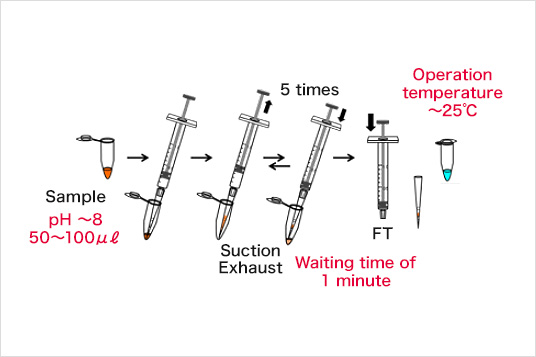
Example of a Thio-tag™ tip experiment
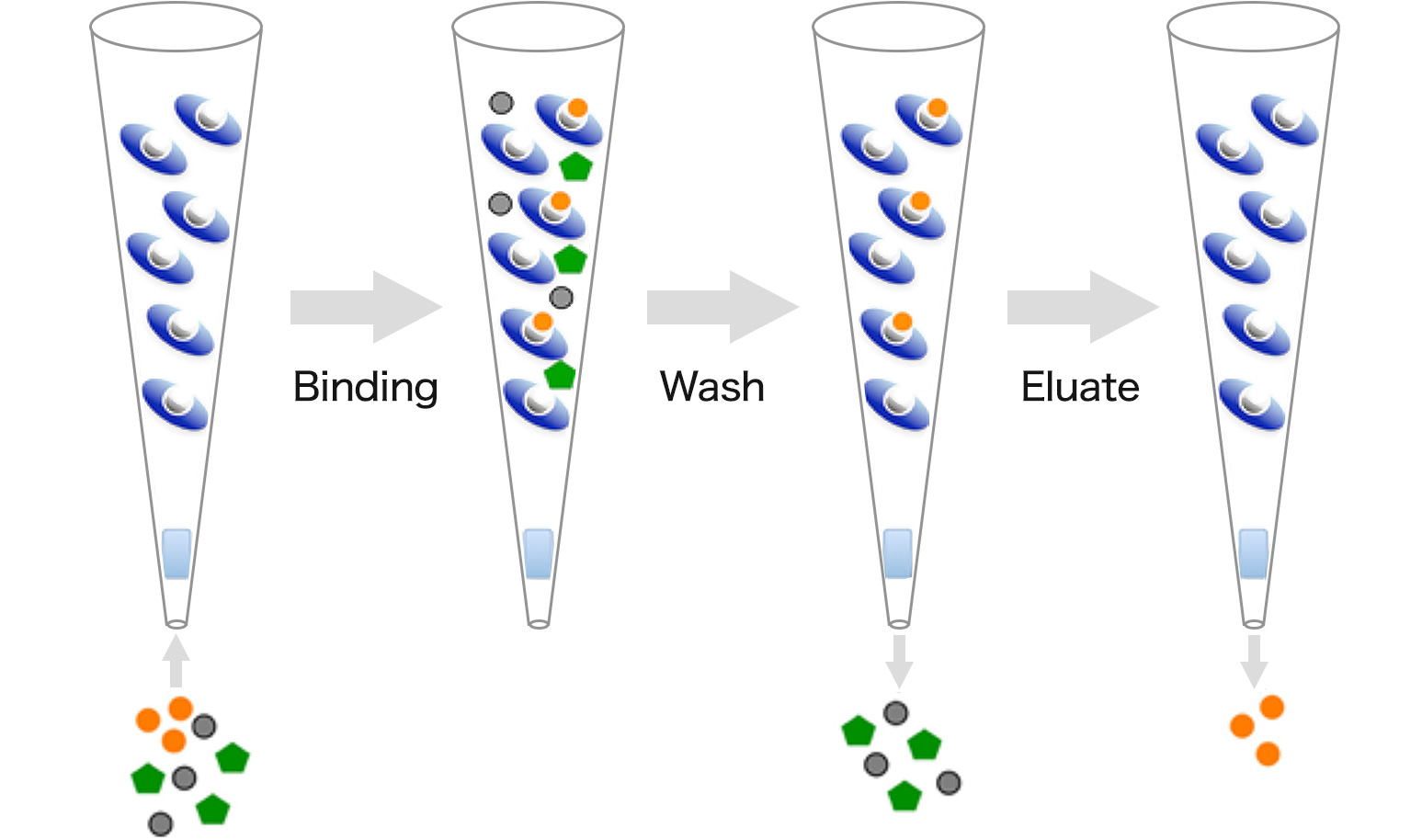
Separation procedure
Binding
Wash
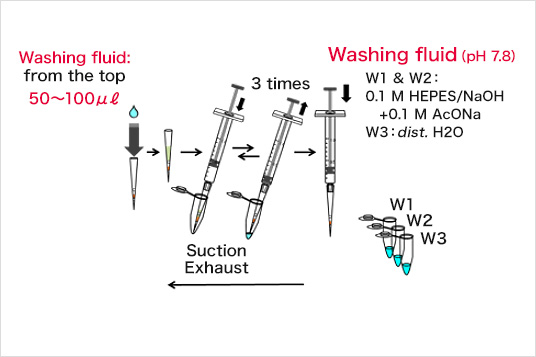
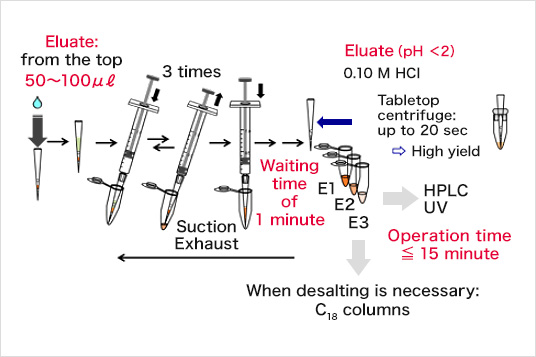
Eluate
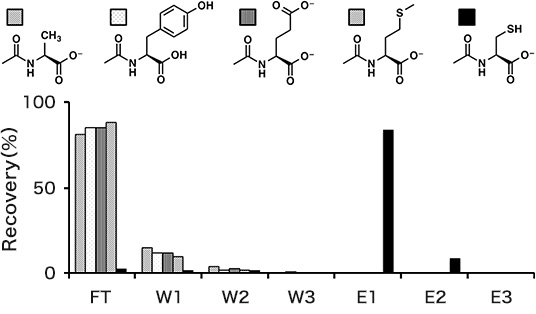
Separation of N-acetylcysteine
- - Sample solution: 20 nmol each of N-acethyl-amino acids in 50 µL of Sol. B
- - Washing solution: Sol. C (50 µL x 2: W1 and W2); distilled water (50 µL: W3)
- - Elution solution: Sol. D (50 µL x 3: E1, E2, and E3)
The separation experiment was conducted according to the basic protocol except using Sol. C for preparation of the sample solution. The recoveries of N-acethyl amino acids are shown in Fig. 1. The quantity in the each fraction was analyzed by reversed phase HPLC. The recovery of N-acethylcysteine in the elution fractions was 93%. The other N-acethyl-amino acids were all eliminated in the FT and W1–W3 fractions.
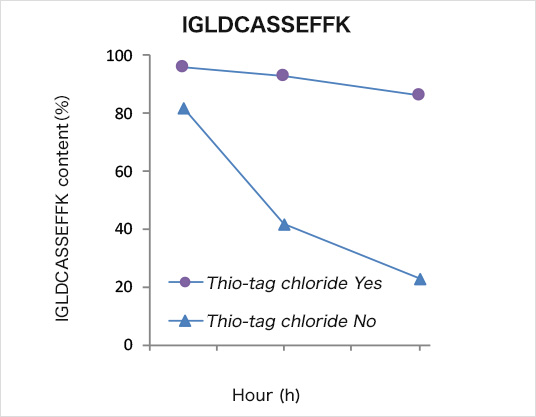
Selective separation of cysteine-containing peptides
- - Sample solution: Tryptic digest of 5 nmol β-casein#
- - + 6 nmol enolase cysteine peptide (ECP) in 50 µL of pH 7.4 HEPES buffer
- - Washing solution: Sol. C (50 µL x 2: W1 and W2); distilled water (50 µL: W3)
- - Elution solution: Sol. D (50 µL x 3: E1, E2, and E3)
The separation experiment was conducted in reference to the basic protocol at room temperature and pH 7.4. The total time for the separation experiment was within 15 min. The separation result was analyzed by a reverse phase HPLC with a gradient mode. The cysteine-containing peptide was preferentially eluted in the E1 fraction at recovery of 74%.
#The β-casein digest has no cysteine-containing peptide. A small amount of a monophosphorylated peptide * from β-casein was eluted in the E1 fraction.
ECP: Ile-Gly-Leu-Asp-Cys-Ala-Ser-Ser-Glu-Phe-Phe-Lys
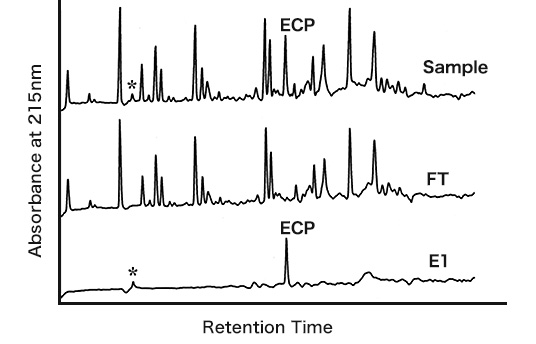
FT is flow-through fraction. E1 is the first elution fraction.
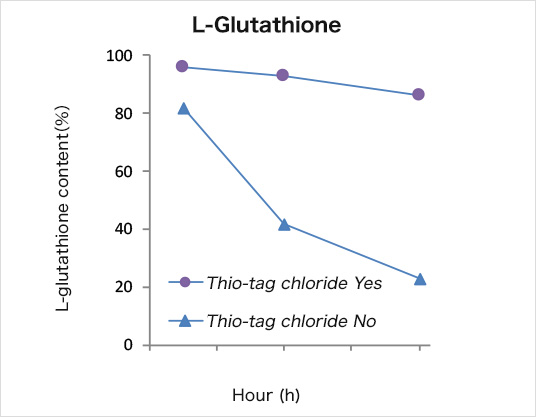
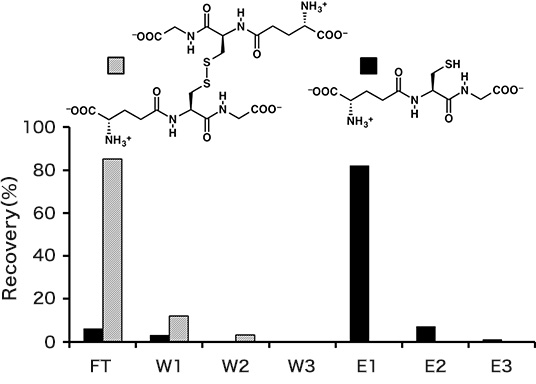
Separation of reduced glutathione
- - Sample solution: 20 nmol of SH-glutathione and 10 nmol of SS-glutathione in 50 µL of Sol. C
- - Washing solution: Sol. C (50 µL x 2: W1 and W2); distilled water (50 µL: W3)
- - Elution solution: Sol. D (50 µL x 3: E1, E2, and E3)
The separation experiment was conducted according to the basic protocol at room temperature except using Sol. C for preparation of the sample solution. The recoveries of reduced and oxidized species are shown in Fig. 2. The quantity in the each fraction was analyzed by reversed phase HPLC. Total recovery of reduced glutathione was 90% in the elution fractions. The SS-form of glutathione was eliminated in the FT and W1–W3 fractions.
Sol. B (Binding buffer) : 0.10 mol/L HEPES–NaOH (pH 7.8)
Sol. C (Washing buffer) : 0.10 mol/L HEPES–NaOH + 0.10 mol/L CH3COONa (pH 7.8)
Sol. D (Elution buffer) : aqueous 0.10 M HCl
References on Thio-tag™ Chemistry:
imple enrichment of thiol-containing biomolecules by using zinc(II)–cyclen-functionalized magnetic beads, Journal of Separation Scienece, 37, 1601-1609 (2014), H. Fujioka, M. Tsunehiro, M. Kawaguchi, Y. Kuramoto, H. Kurosaki, Y. Hieda, E. Kinoshita-Kikuta, E. Kinoshita, and T. Koike
Role of Zinc(II) in β-Lactamase II: A Model Study with a Zinc(II)-Macrocyclic
Tetraamine (1,4,7,10-Tetraazacyclododecane, Cyclen) Complex, Journal of American Chemical Society, 116, 8443-8449 (1994), T. Koike, M. Takamura, and E. Kimura
Related contents
Inquiries about products and technologies
Please contact us for consultations and inquiries regarding products and technologies.