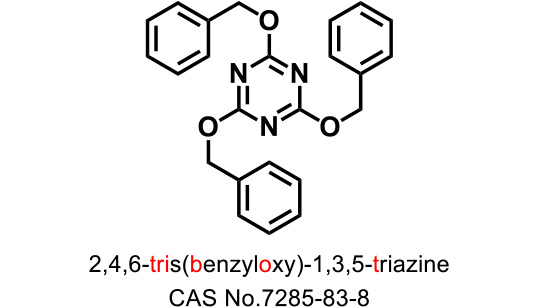
Triazine-type hydroxy-group protecting agents
Williamson ether synthesis is the benzylation of hydroxyl groups under basic conditions. Benzylation under neutral or acidic conditions is effective for substrates to which Williamson ether synthesis cannot be applied. Our company offers a line of benzylation agents that can be used under neutral or acidic conditions.
We also accept orders for contract manufacturing that uses such special benzylation agents. Please contact us for consultation.
* We handle these protecting agents and methodologies under a licensing contract with Kanazawa University (Professor Munetaka Kunishima, Faculty of Pharmacy, Institute of Medical, Pharmaceutical and Health Sciences), which is the applicant of patent submission.
TriBOT and TriBOT-PM
The TriBOT series is used in the presence of acidic catalyst. It can also be used for substrates that are unusable under basic conditions. In addition, the TriBOT series is stable in moist air and easy to handle without the lachrymatory or irritating effects of benzyl chloride or benzyl bromide.
These products are available for bulk supply for continuous usage after commercialization.
Main features of the TriBOT series
| Stability in the presence of water or air allows for easy handling. |
| No lachrymatory or irritating effects. Negative in the Ames test. |
| Superior binding to functional groups, allowing for high yields. |
| Three intramolecular benzyl groups contribute to reactions, showing excellent atom economy. |
TriBOT is a benzylation agent that mainly uses trifluoromethanesulfonic acid (TfOH) as a catalyst.
Reactions proceed effectively in the presence of 1,2-dimethoxyethane or 1,4-dioxane at room temperature.
Reaction example:
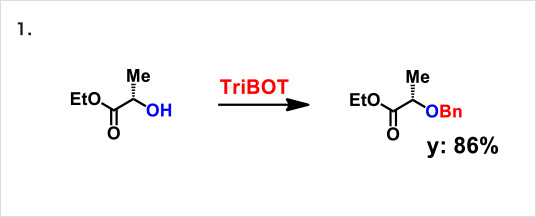
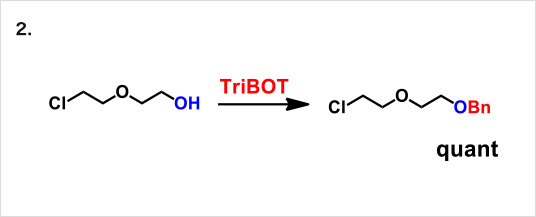
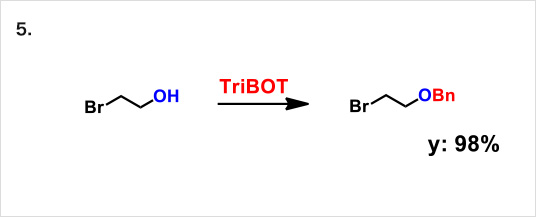
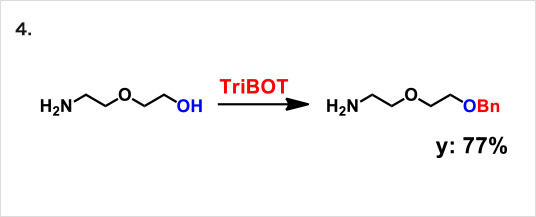
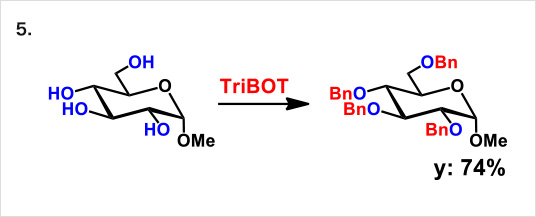
* Reference: Org. Lett., 2012, 14 (19), pp 5026–5029
| Code No. | Product | Content | Suggested retail price (JPY) |
|---|---|---|---|
| 385-02182 | TriBOT 2,4,6-tris(benzyloxy)-1,3,5-triazine |
25g | 12,000 |
| 387-02181 | 100g | 36,000 |
* This product was developed by Professor Munetaka Kunishima, Faculty of Pharmacy, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University
* No. of the granted patent: 6069783
Purchase inquiries
Please contact FUJIFILM Wako Pure Chemical Corporation for inquiries regarding the purchase of this product.
Benzyl protecting reagent, organic synthesis, FUJIFILM Wako Pure Chemical Corporation:
https://labchem-wako.fujifilm.com/us/category/00100.html
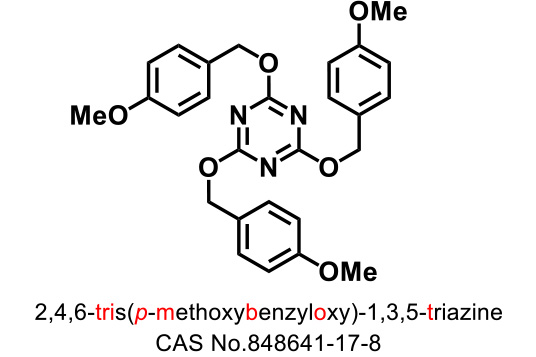
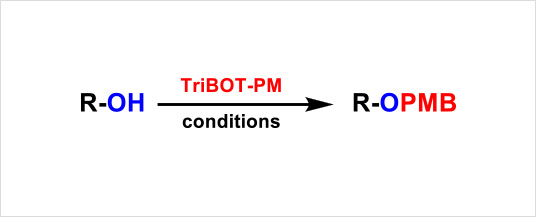
TriBOT-PM is a para-methoxybenzylation (PMB) agent used in the presence of an acidic catalyst. When compared with TriBOT, the reaction proceeds under mild conditions, allowing for flexibility in choosing acidic catalysts and reaction solvents depending on the substrate.
| Usable acidic catalysts | Usable reaction solvent systems |
|---|---|
| TsOH, CSA, BF3・Et2O, TfOH, etc. | AcOEt, THF, DME, dioxane, CH2Cl2/Et2O, etc. |
Reaction example:
| Product | TriBOT-PM (equiv.) |
Conditions acid (mol%), solvant, temp, time |
Yield | |
|---|---|---|---|---|
| 1. | 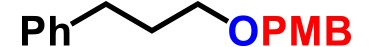 |
0.37 | TfOH (0.3), AcOEt, r.t., 15min | 94% |
| 2. | 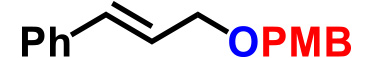 |
0.65 | CSA (10), THF, reflux, 11hr | 85% |
| 3. | 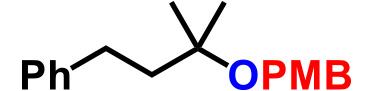 |
2.0 | CSA (15), THF, reflux, 8hr | 94% |
| 4. | 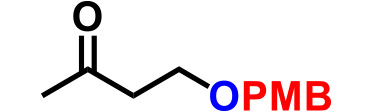 |
0.6 | CSA (10), THF, reflux, 6hr | 83% |
| 5. | 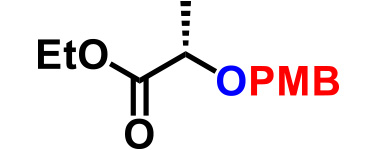 |
1.1 | CSA (15), THF, reflux, 13hr | 87% |
| 6. | 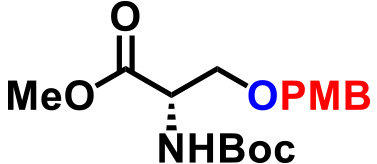 |
1.0 | CSA (15), THF, reflux, 9hr | 77% |
| 7. | 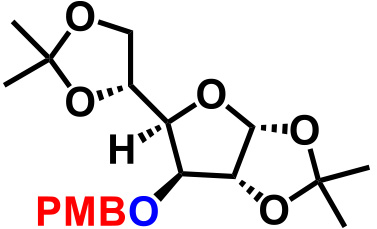 |
1.1 | CSA (15), THF, reflux, 7.5hr | 90% |
| 8. | 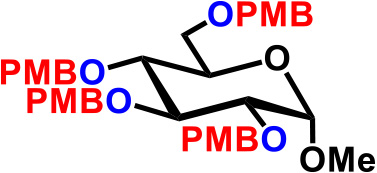 |
4.8 | CSA (60), THF, reflux, 30hr | 69% |
* Reference: Synthesis 2013, 45, 2989-2997.
| Code No. | Product | Content | Suggested retail price (JPY) |
|---|---|---|---|
| 381-02601 | TriBOT-PM 2,4,6-tris(p-methoxybenzyloxy)-1,3,5-triazine |
10g | 9,000 |
| 387-02603 | 50g | 27,500 |
* This product was developed by Professor Munetaka Kunishima, Faculty of Pharmacy, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University
* No. of the granted patent: 6069783
Purchase inquiries
Please contact FUJIFILM Wako Pure Chemical Corporation for inquiries regarding the purchase of this product.
Benzyl protecting reagent, organic synthesis, FUJIFILM Wako Pure Chemical Corporation:
https://labchem.wako-chem.co.jp/products/000101/
TriBOT-PM is a para-methoxybenzylation (PMB) agent used in the presence of an acidic catalyst. When compared with TriBOT, the reaction proceeds under mild conditions, allowing for flexibility in choosing acidic catalysts and reaction solvents depending on the substrate.
Features
| No catalyst is required. The reaction proceeds under neutral conditions. | Only to be mixed at room temperature. |
| Superior in binding to functional groups, allowing for high yields. | Easy to handle with no lachrymatory or irritating effects. |
Reaction example:
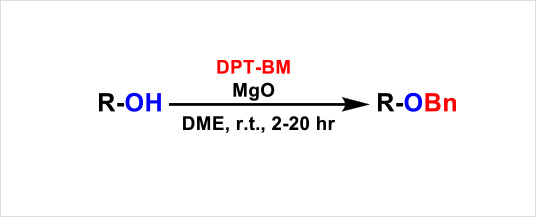
| Product | DPT-BM (equiv.) |
Time | Yield | |
|---|---|---|---|---|
| 1. | 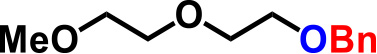 |
2.0 | 2hr | 97% |
| 2. | 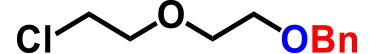 |
2.0 | 2hr | 97% |
| 3. | 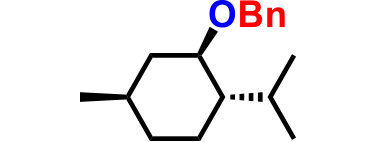 |
2.0 | 2hr | 86% |
| 4. | 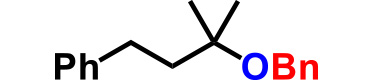 |
3.0 | 4.5hr | 78% |
| 5. | 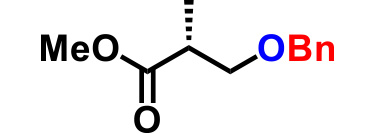 |
2.0 | 2hr | 82% |
| 6. | 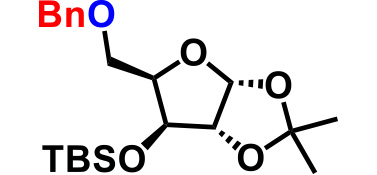 |
3.0 | 2hr | 81% |
| 7. | 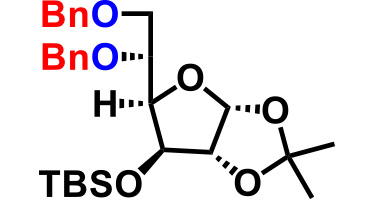 |
8.0 | 20hr | 64% |
* Reference: Chemistry - A European Journal, 2014, 20, 12274-12278
| Code No. | Product | Content | Suggested retail price (JPY) |
|---|---|---|---|
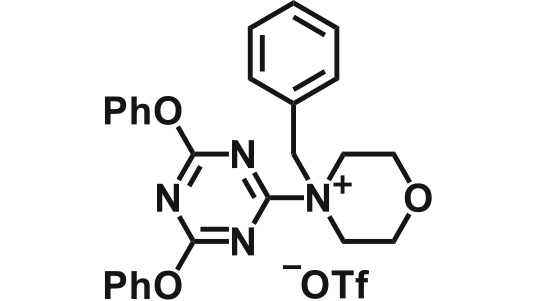
| 389-07923 | DPT-BM 4-(4,6-diphenoxy-1,3,5-triazin-2-yl)-4-benzylmorpholinium trifluoromethanesulfonate |
1g | 18,000 |
| 383-07921 | 5g | 86,400 |
* This product was developed by Professor Munetaka Kunishima, Faculty of Pharmacy, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University
* No. of the granted patent: 5896407
Purchase inquiries
Please contact FUJIFILM Wako Pure Chemical Corporation for inquiries regarding the purchase of this product.
Benzyl protecting reagent, organic synthesis, FUJIFILM Wako Pure Chemical Corporation:
https://labchem.wako-chem.co.jp/products/000101/
Related contents
Inquiries about products and technologies
Please contact us for consultations and inquiries regarding products and technologies.