Compound library synthesis
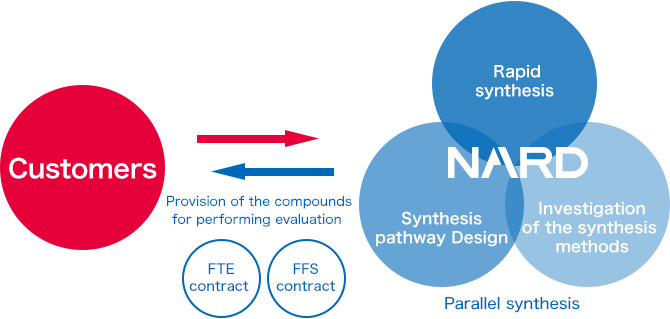
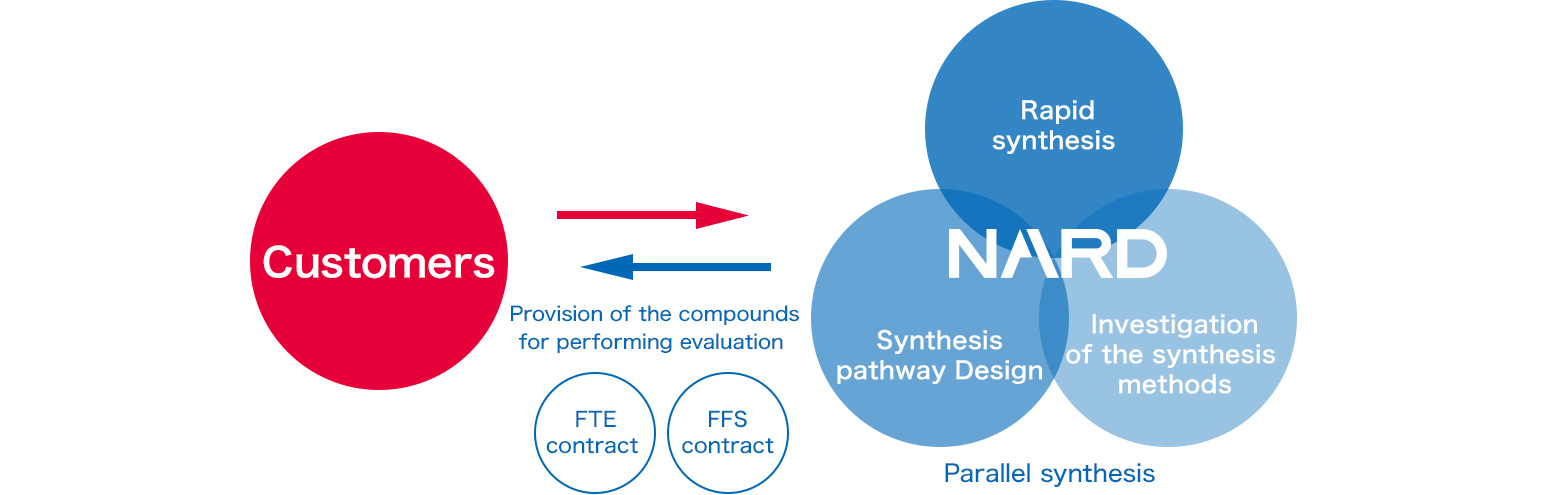
Based on the existing synthesis methods, we utilize our expertise to propose processes suitable for performing multi-sample synthesis and provide services that are applicable to various processes, ranging from synthetic design to reaction operation and purification.
We tailor our sample shipments according to our customers' requirements; the samples are delivered in a state appropriate for conducting planned experiments.

Disclosure of candidate compounds (optimization of the candidate compounds)
-
1We propose a multi-sample synthetic process based on ordinary organic synthesis.
We conduct studies related to parallel liquid-phase synthesis, solid-state synthesis, purification methods, and proposal of synthetic pathways.
-
2Library Synthesis
Proceeding with production in accordance with the efficiently optimized synthetic processes.
-
3Library Purification
Improving the product quality by purification using liquid-phase preparatory equipment, solid-phase reagents, MS-triggered chromatography systems, etc.
-
4Quality Control
Structural and purity analyses of the samples are conducted using LC/MS, NMR, and HPLC. Samples are delivered in the requested form.
-
5Delivery
Library synthesis reaction: example 1
Example1: amidation
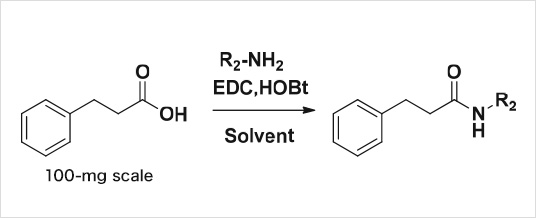
| Delivered amount | 20-mg plus minimum with more than 90% purity 1000 compounds/3 months A reaction success rate of more than 90% |
|---|
Example2: Suzuki coupling
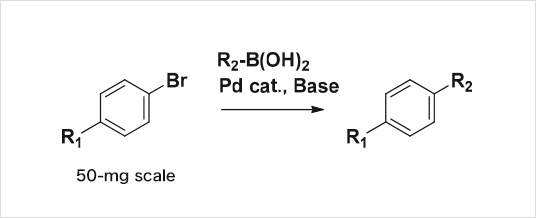
| Delivered amount | 10-mg plus minimum with more than 95% purity 100 compounds/1 month A reaction success rate of more than 90% |
|---|

100 compounds / day

100 compounds / day
Library synthesis reaction: example 2
| Suzuki coupling | Buchwald amination | Heck reaction | Stille coupling |
| Sonogashira reaction | Ullmann reaction | Negishi coupling | Mitsunobu reaction |
| Mannich reaction | Wittig reaction | HWE reaction | Grignard reaction |
| Gabriel synthesis | Jones Oxidation | Michael Addition | Simmons-Smith cyclopropanation |
| Williamson Ether synthesis | Heterocyclic synthesis | Pd/C H2 | NaBH4 reduction |
| LiAlH4 reduction | Condensation reagent | etc … |
Library synthesis results based on the FTE contract
Short-term contract (1 to 2 projects)
Synthesis of 100–400 compounds per month (including library synthesis study)

Long-term contract (multiple projects)
Synthesis of 150–400 compounds per month (including library synthesis study)
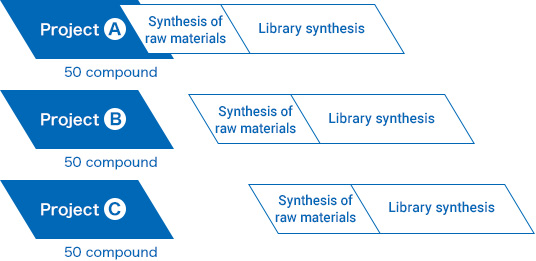
The flow of basic FFS contracts
-
1
Library synthesis
Preliminary study -
2
Library synthesis
-
3
LC–MS separation
Purification -
4
QC(LC-MS)
Basic conditions for conducting separation and analysis
| Column | Method |
|---|---|
| YMC Triat C18, 5µm 4.6mm×50mm |
Flow velocity: 2 mL/min Temperature: Room temperature Gradient conditions |
| Min | 0.1% TFA in H2O | CH3CN |
|---|---|---|
| 0 | 95 | 5 |
| 0.5 | 95 | 5 |
| 3 | 5 | 95 |
| 5 | 5 | 95 |
| Column | Method |
|---|---|
| YMC-Actus Triat C18, 5µm 150mm×30mm |
Flow velocity: 60 ml/min Temperature: Room temperature Gradient conditions |
| Min | 0.1% TFA in H2O | CH3CN |
|---|---|---|
| 0 | 95 | 5 |
| 1 | 95 | 5 |
| 11 | 20 | 80 |
| 12 | 5 | 95 |
Main equipment for conducting parallel synthesis
| Reactors and equipment for conducting parallel synthesis | |
|---|---|
| Parallel reaction equipment 20 mL, 13 chambers (-78℃ to 150℃) 150 mL, 6 chambers (-78℃ to 150℃) |
Solvent concentrating equipment (Centrifugal concentration, air dry) |
| Parallel reaction equipment 20 mL, 50 chambers(0℃ to 150℃) |
Parallel divided injection technique |
| Parallel reaction equipment 2 mL, 96 chambers |
|
| Purification | Analysis |
|---|---|
| Automated preparative column chromatography (SiO2, NH type, ODS type, etc.) |
NMR (400MHz) |
| MS-triggered automated preparative chromatography system × 2 | High-performance liquid chromatography, HPLC (PDA, UV) |
| Solid-state reaction purification techniques | Multi-sample automated measurement system (LC/MS) (PDA, ELSD) |
Related contents
Inquiries about products and technologies
Please contact us for consultations and inquiries regarding products and technologies.